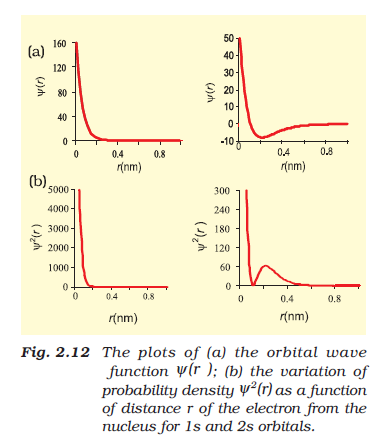
This gives fairly good representation of the shapes of the orbitals.
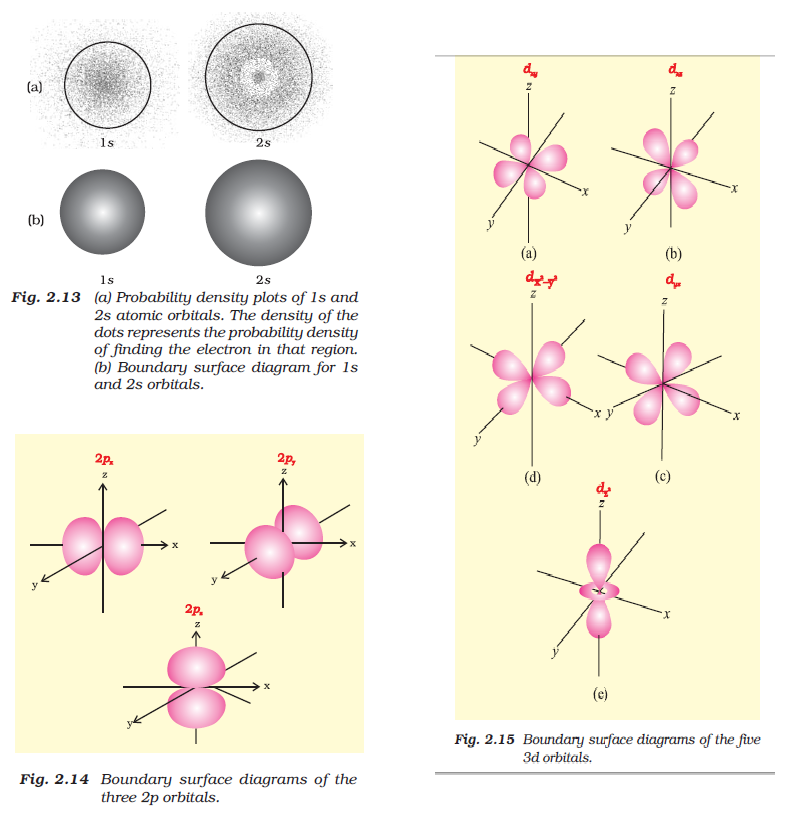
In this, a boundary surface or contour surface is drawn in space for an orbital on which the value `|psi|^2 ` is constant.
Definition : For a given orbital, only that boundary surface diagram of constant probability density is taken which encloses a region or volume in which the probability of finding the electron is `90%`. fig [2.13 (b)].
Note : - We do not draw boundary surface diagram of `100%` probability because ` psi^2` has always some value at any finite distance from the nuclues, so it is not possible to draw a boundary surface diagram of a rigid size of `100%` probability.
For `s`-orbital, boundary surface diagram is spherical in shape. i.e the probability of finding the electron in all direction is equal.
Size of `s`-orbital `prop 'n'`
For `p`-orbital (Fig 2.14), the boundary surface diagram is not spherical. But it has two lobes which are on either side of the plane passing through the nucleus. At this plane, probability density function is zero.
The shape, size and energy of the three orbitals are identical. The lobes lie along the x, y and z axis and they are given the designations `2p_x`, `2p_y` and `2p_z`.
For `p`-orbital, the energy of `p`-orbitals `prop n`
For `d`-orbitals, `l = 2`. And there are five `d`-orbitals, designated as `d_(xy), d_(yz), d_(zx), d_(x^2-y^2)` and `d_(z^2)`. See fig 2.15.
All the five `d`-orbitals have same energy.
This gives fairly good representation of the shapes of the orbitals.
In this, a boundary surface or contour surface is drawn in space for an orbital on which the value `|psi|^2 ` is constant.
Definition : For a given orbital, only that boundary surface diagram of constant probability density is taken which encloses a region or volume in which the probability of finding the electron is `90%`. fig [2.13 (b)].
Note : - We do not draw boundary surface diagram of `100%` probability because ` psi^2` has always some value at any finite distance from the nuclues, so it is not possible to draw a boundary surface diagram of a rigid size of `100%` probability.
For `s`-orbital, boundary surface diagram is spherical in shape. i.e the probability of finding the electron in all direction is equal.
Size of `s`-orbital `prop 'n'`
For `p`-orbital (Fig 2.14), the boundary surface diagram is not spherical. But it has two lobes which are on either side of the plane passing through the nucleus. At this plane, probability density function is zero.
The shape, size and energy of the three orbitals are identical. The lobes lie along the x, y and z axis and they are given the designations `2p_x`, `2p_y` and `2p_z`.
For `p`-orbital, the energy of `p`-orbitals `prop n`
For `d`-orbitals, `l = 2`. And there are five `d`-orbitals, designated as `d_(xy), d_(yz), d_(zx), d_(x^2-y^2)` and `d_(z^2)`. See fig 2.15.
All the five `d`-orbitals have same energy.